Our Diabetes and the Islet program aims to better understand the loss of function and survival of pancreatic islet cells that characterizes all forms of diabetes. Our discoveries will help develop better therapies for people living with diabetes, with our ultimate goal being to find ways to prevent the disease.
Over 30 million people in the U.S. are affected by diabetes, and many have pre-diabetes or do not yet know they have diabetes. Diabetes is mainly classified as Type 1 or Type 2 diabetes, and affect children, youth and adults. All forms of diabetes are diagnosed based on high blood glucose (sugar) levels, and result in long-term complications, including eye, kidney and cardiovascular complications.
The pancreatic islet is the site of insulin production, a hormone critical for the control of blood glucose. Defects in islet function are therefore central to diabetes development, and this process involves several of the cell types found within the islet. Understanding how and why the islet fails in diabetes is the driving force behind our research.
What is an Islet
The pancreatic islet is a mini organ comprising multiple different types of cells. Proper functioning of all these cell types is required to maintain blood glucose levels in the normal range
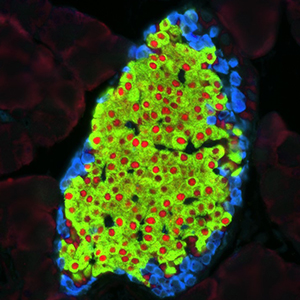
Anatomy of an Islet
Defects in the function and survival of the multiple cell types that make up the islet are fundamental to all types of diabetes

































































Our program faculty bring diverse expertise to the problem of understanding the islet and the mechanisms by which it fails in diabetes. We cover a broad spectrum of research, including studies to interrogating fundamental islet biology, dissection of cellular mechanisms underlying islet failure and groundbreaking clinical research.
FUNDAMENTAL ISLET BIOLOGY
Furthering our knowledge of fundamental islet biology is key to better understanding what goes wrong in diabetes and how to fix it. Insulin release is required for normal glucose homeostasis, and is deficient in all forms of diabetes. Several program members are uncovering novel pathways and mechanisms governing the complex process of insulin release under normal conditions. Their findings provide new targets for therapeutic development. Beta-cell loss occurs in all forms of diabetes. Members of this program are therefore working to develop strategies to promote of beta cell regeneration and to provide sources of new beta cells for transplantation. An emerging area of interest in the field is that the beta cell requires interactions with several other islet cell types to maintain optimal health and function. Multiple program members are focused on this area, investigating how interactions between beta cells and alpha cells, immune cells, islet blood vessels and the associated extracellular matrix all contribute to normal islet health and function.
CELLULAR MECHANISMS OF ISLET FAILURE
The diabetic islet exists within a hostile environment, which provides some unique challenges for its survival and function. We are studying how islet autoimmunity results in type 1 diabetes, including the role of auto-antibodies in this process. Several program members study islet amyloid, a feature of all types of diabetes, and how it exerts its toxic effects on (and through) multiple islet cell types. The role of peptidases, including neprilysin, in modulating islet function in diabetes and the impact of islet endothelial dysfunction on beta-cell function/survival are also active areas of research. Together, these research efforts allow us to probe mechanisms underlying islet failure and pathology in multiple forms of diabetes, with the goal of finding new approaches for treatment and prevention.
CLINICAL RESEARCH
Clinical Research within our programs encompasses several areas. Our faculty are involved in major national clinical trials in prediabetes, type 1 and type 2 diabetes. Local studies are focused on pharmacological therapies and diabetes technology. Our faculty’s expertise is helping the research and clinical communities better understand glucose metabolism abnormalities that occur in diseases such as cystic fibrosis.
Fundamental Islet Biology Investigators


Michael Ailion, PhD
View Department Profile
Dr. Ailion’s laboratory seeks to identify proteins important for the biogenesis of insulin secretory granules and determining their cellular mechanism of action. Additionally, they are testing whether mutations in these proteins may lead to diabetes in mammalian systems.


Vincenzo Cirulli, MD, PhD
View Profile
Associate Professor of Medicine
Division of Metabolism, Endocrinology and Nutrition


Laura Crisa, MD, PhD
View Profile
Associate Professor of Medicine
Division of Metabolism, Endocrinology and Nutrition


Cole DeForest, PhD
View Department Profile
The DeForest Group seeks to integrate the governing principles of rational design with fundamental concepts from material science, synthetic chemistry, and stem cell biology to conceptualize, create, and exploit next-generation materials to address a variety of health-related problems. We are developing new classes of user-programmable hydrogels whose biochemical and biophysical properties can be tuned in time and space over a variety of scales. In partnership with the UWMDI, we are exploring the utility of these systems in treatment of diabetes.


Ian Sweet, PhD
View Profile
Associate Professor of Medicine
Division of Metabolism, Endocrinology and Nutrition
Cellular Mechanisms of Diabetes Investigators


Christiane Hampe, PhD
View Profile
Associate Professor of Medicine
Division of Metabolism, Endocrinology and Nutrition


Rebecca Hull, PhD
View Profile
Director, Islet and Diabetes Program, UW Diabetes Institute
Associate Professor of Medicine
Division of Metabolism, Endocrinology and Nutrition


Thomas Wight, PhD
View Department Profile
The Wight lab focuses on the role of the extracellular matrix (ECM), and particulalry the ECM components hyaluronan (HA) and proteoglycans, in the regulation of cell phenotype in diseases such as diabetes, rheumatoid arthritis, cancer, lung and cardiovascular disease.


Sakeneh Zraika, PhD
View Department Profile
The Zraika lab studies the pathophysiology of type 2 diabetes, with an emphasis on the islet lesion that characterizes the disease. In this context, a major focus is elucidating the role of the protein neprilysin, in modulating insulin secretion, beta-cell mass and glucose homeostasis.
Clinical Research Investigators


Edward Boyko, MD, MPH
View Department Profile
My research program focuses on the epidemiology type 2 diabetes and its complications, associated metabolic disorders, and obesity. I have conducted research on the role of body composition in the development of type 2 diabetes and related conditions such as hypertension and metabolic syndrome. My interest in diabetes complications has focused on defining risk factors for diabetic foot ulcer and lower limb amputation.


Jane Buckner, MD
View Department Profile
Dr. Buckner’s research is focused on identifying the underlying the cellular and molecular mechanisms responsible for the dysregulation of the adaptive immune response in the setting of human autoimmune disease. Ongoing T1D projects in her lab are focused on 1) defining the mechanisms by which cytokine signaling pathways are altered in diabetes, and how this affects T cell function; 2) determining the functional and synergistic impact of genetic variants associated with type 1 diabetes on the adaptive immune response; and 3) developing regulatory T cell-based therapies for the prevention and treatment of T1D.


William Hagopian, MD, PhD
View Department Profile
Dr Hagopian researches human Type 1 diabetes, from immunogenetic biomarkers to predict and follow pathogenesis, to environmental triggers of disease, to immunotherapies to ameliorate disease. These can be used in large pathogenesis studies funded by NIH, in population-based public health approaches, in NIH or pharma-sponsored prevention trials, and for classification of risk or diagnosis in individuals.


Steven Kahn, MB, ChB
View Department Profile
Dr. Kahn's basic science studies focus on the role of islet amyloid formation in the pathogenesis of the ßcell loss observed in type 2 diabetes and islet transplantation.


Jerry Palmer, MD
View Department Profile
Dr. Palmer’s primary research focus is on Type 1 Diabetes. He directs the UW DCCT/EDIC clinical center and actively participates in the Type 1 Diabetes TrialNet international consortium. His laboratory is investigating the importance of islet autoimmunity in patients with phenotypic type 2 diabetes.


Cate Pihoker, MD
View Department Profile
Dr. Pihoker's research interests include investigating factors that predict the clinical course of diabetes, diabetes typology and improving outcomes of children with diabetes.


Dace Trence, MD
View Department Profile
Research interests include clinical application of new
technology in the self-management of diabetes mellitus, application of new pharmacologic tools in the management of diabetes mellitus, and education from patient to experienced clinician level in chronic disease management.


Kristina Utzschneider, MD
View Department Profile
My research career has been devoted to clinical investigation of the pathogenesis of type 2 diabetes and related conditions such as non-alcoholic fatty liver disease. I have been involved in multiple clinical research studies aimed at investigating ß-cell dysfunction and insulin sensitivity in people at risk for diabetes using both dietary and medication interventions. These studies have utilized oral and intravenous glucose tolerance tests, hyperinsulinemic-euglycemic clamps and mathematical modeling techniques to estimate insulin sensitivity and ß-cell function.

