Discovering new brain-based treatments for obesity and diabetes.
Obesity remains a leading public health problem due to its association with diabetes, heart disease, cancer and other significant chronic illnesses. Currently, over 2/3 of US adults are overweight or obese, perpetuated by an environment that promotes unhealthy eating habits and sedentary behavior. Unfortunately, over the twenty years since the discovery of the adiposity hormone leptin and the characterization of its action in the hypothalamus to regulate energy homeostasis, few effective obesity treatments have been developed. This failure of progress highlights the difficulties inherent in a “neurocentric” approach to pharmacotherapy (limited CNS bioavailability, undesired neuropsychiatric side effects, and neural network compensation). Thus, new approaches to obesity pathogenesis are urgently needed.
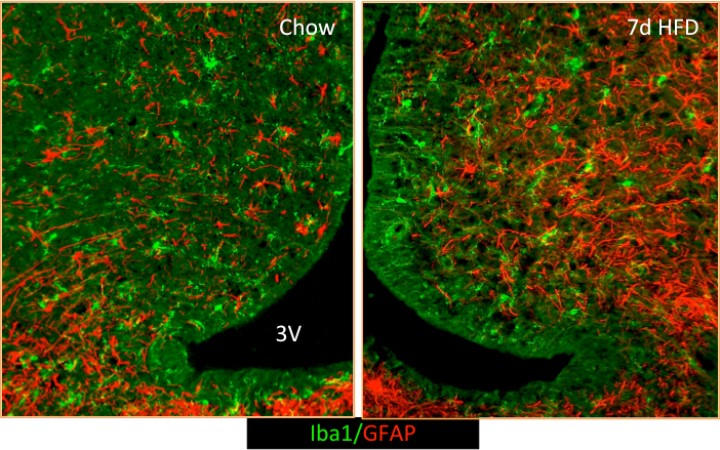
Recently, we found that the onset of diet-induced obesity (DIO) in male rodents is associated with hypothalamic inflammation and activation of surrounding astrocytes and microglia (resident CNS macrophages) (Thaler JCI 2012). Our current research focuses on determining the role of the gliosis process in triggering neuronal stress and providing a root cause of high fat diet (HFD)-induced weight gain. We have a variety of ongoing projects investigating the importance of non-neuronal cells in energy homeostasis.
1) Female rodents are relatively DIO-resistant and develop neither hypothalamic inflammation nor brain immune cell (microglia) activation. This protection requires the microglial silencing CX3CL1 receptor (CX3CR1), with CX3CR1 KO females developing DIO and hypothalamic microglial activation like males. Conversely, males treated with CX3CL1 show reduced diet-associated weight gain and microglial activation like females. Together, these data suggest that the CX3CL1-CX3CR1 microglial signaling system can be targeted to reduce DIO susceptibility, support a role for HFD-induced microglial inflammatory activation in obesity pathogenesis, and provide evidence for sexually dimorphic involvement of microglia in metabolism (Dorfman et al, Nature Communications 2017). We are currently 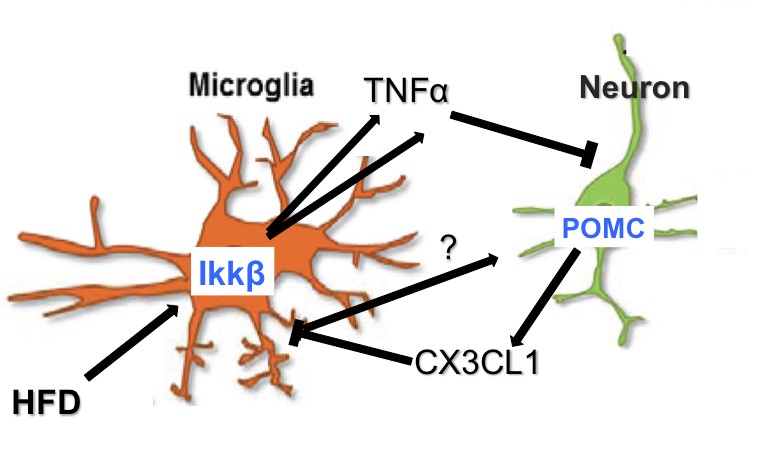 extending these findings by characterizing microglial-neuron crosstalk through CX3CL1-CX3CR1 signaling. In addition, we are pursuing the molecular mechanisms underlying sex differences in gliosis focusing on the role of estrogen receptors in microglial inflammatory activation and, in a productive collaboration with Dr. Ellen Schur, the impact of androgen signaling on gliosis and obesity susceptibility.
extending these findings by characterizing microglial-neuron crosstalk through CX3CL1-CX3CR1 signaling. In addition, we are pursuing the molecular mechanisms underlying sex differences in gliosis focusing on the role of estrogen receptors in microglial inflammatory activation and, in a productive collaboration with Dr. Ellen Schur, the impact of androgen signaling on gliosis and obesity susceptibility.
2) Hypothalamic inflammation contributes to DIO susceptibility but the source and cell types involved in this inflammation remain poorly understood. Using cutting edge genetic and pharmacological tools, we recently demonstrated that both microglial and astrocytic inflammation via the NF-κB/Ikkβ pathway are required for hyperphagia and weight gain during high fat diet-induced obesity (Douglass et al., Molecular Metabolism 2017; Valdearcos, Douglass, et al. Cell Metabolism 2017). We are now using transcriptomic and candidate-based approaches to elucidate the molecular pathway involved in triggering microglial activation and identify the microglial gene products that effect changes in energy balance.
3) Our previous work describing the role of glial activation in obesity pathogenesis revealed an unexpected divergence in function between microglia and astrocyte inflammatory signaling with regard to glucose metabolism. While astrocyte Ikkβ knockout mice have improved glucose tolerance as expected in thinner animals, microglial Ikkβ knockout mice remained unprotected from obesity-associated glucose dysregulation. Using a novel chemogenetic mouse model, pair-feeding studies, and pharmacological approaches, we have discovered that microglial activation improves glucose handling even while promoting weight gain. We are currently determining the molecular mechanism underlying this surprising finding with the hopes of developing novel diabetes therapeutics.
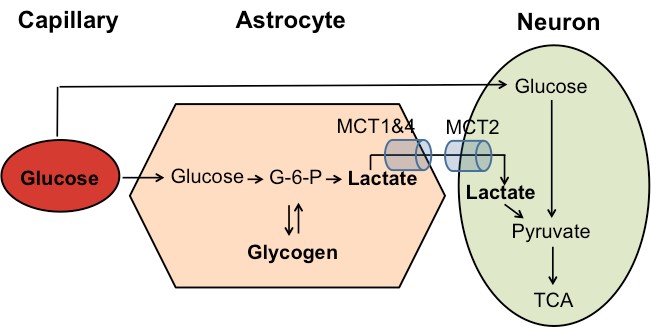
4) Astrocytes provide fuel substrates for synaptic activity through the glycogenolysis-glycolysis-lactate pathway. We have studied real-time lactate fluctuations in the hypothalamus during feeding and in response to glycogenolysis inhibitors. Current work aims to identify the functional role of astrocyte-derived lactate in feeding behavior using novel chemogenetic and pharmacological methods.
Our work is funded by the National Institutes of Health, the American Heart Association, the American Diabetes Association, and Novo Nordisk USA.
Current Members of the Thaler Laboratory

Joshua Thaler, MD, PhD
Associate Professor

Mauricio Dorfman, PhD
Research Assistant Professor

Jeremy Frey, BS
Research Scientist

Anzela Niraula, PhD
Postdoctoral Fellow

Kelly Ness, PhD
Postdoctoral Fellow
Alumni Laboratory Members

Rachel Fasnatch, MS
Research Scientist

Jineta Banerjee, PhD
Postdoctoral Fellow

Fernando Lara Lince, MD
Laboratory Volunteer

Jenny Li, PhD
Visition Scientist

John Douglass, PhD
Postdoctoral Fellow

Alice Wyse Jackson
Postdoctoral Fellow
Contact us
UW Medicine Diabetes Institute
750 Republican Street, Box 358062
Seattle, WA 98109
Josh Thaler: (206) 897-1802
Laboratory: (206) 616-2482
Fax: (206) 897-5293
Joshua Thaler: jpthaler@uw.edu
Mauricio Dorfman: dorfmanm@uw.edu
Jeremy Frey: freyj@uw.edu
To inquire about Postdoctoral and Graduate Student Openings, email jpthaler@uw.edu




